Potential Protective Effect of Sickle Cell Gene Allele on HIV Infection-Juniper Publishers
Juniper Publishers-Journal of Pediatrics
Short Communication
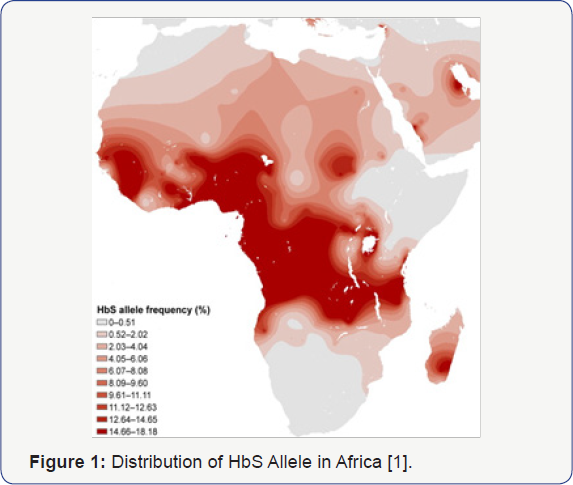
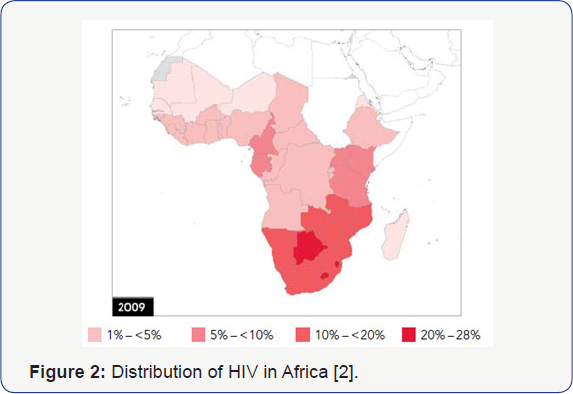
Both Sickle Cell Disease and HIV Infection have
overlapping worldwide distributions, with Africa being the epicenter for
these two conditions (Figure 1 & 2) [1,2].
Sickle Cell Disease is a genetic condition affecting chromosome 11 in
which homozygous recessive individuals will have significant negative
consequences secondary to sickling phenomenon of their red blood cells,
while those that are heterozygous have a survival advantage based on a
well-described resistance to malaria [3].
The Human Immunodeficiency Virus takes advantage of surface proteins on
white cells, resulting in lytic destruction and consequent CD4+
lymphopenia and immunosuppression [4].
Both diseases have significant morbidity and mortality, with limited
treatment options. Nevertheless, observations in our pediatric HIV
program in Newark, NJ, one of the first epicenters for Perinatal HIV
Infection (PHIV), suggested that children with sickle cell disease
infrequently were infected with HIV despite being from a high-risk
population5 (Table 1) [5].
Current understanding of the interplay of genetic impact on surface
membranes supports the idea that genetic variations might impact
susceptibility to infectious diseases. Similar to its protective effect
against malaria, sickle cell trait may provide protection against
acquisition of HIV infection through changes in CXCR4 and CCR5 receptors
on CD4+ lymphocytes, possibly blocking viral entry and infection [6].
Potential mechanisms for this disparity might provide better
understanding and approaches to HIV infection based on sickle cell
status.

The authors have had a long standing hypothesis that
the Sickle Cell Gene Allele (SCGA) may confer a protective effect
against acquisition and/or progression of HIV infection. Rutgers NJMS in
Newark, NJ has long been a center of PHIV care and research since the
onset of the HIV pandemic, developing domestic and global clinical,
educational and research expertise in PHIV infection leading to the
control and subsequent prevention of PHIV in the US. Among our large
population of PHIV African American children, we noted a lower than
expected occurrence of Sickle Cell Hemoglobinopathy (SCH) suggesting
relative protection against PHIV acquisition, and by inference,
progression. However, though the data from the Newark PHIV program is
suggestive of the potential protective effect of the SCGA, it lacks the
patient base needed to achieve sufficient statistical power to confirm
this potential association [5].
Sub-Saharan Africa has approximately 64% of the world's population
living with HIV, 91% of children under 15 years of age living with HIV,
and approximately 10-40% of the world's SCD population [1,7].
The population distribution of individuals in Africa with HbS allele
and HIV are seemingly non-overlapping, supporting preliminary data from
our pediatric cohort [1,2,5].
This non-overlapping distribution is unexpected, given the high
prevalence of both Sickle Cell Disease and HIV in Africa. South Africa,
having a low distribution of SCGA has one of the higher concentrations
of HIV infectivity in Africa, confirming the significance of these
observations. These findings should stimulate further investigation into
the molecular basis for this protective effect, which could influence
the direction of new therapies (drug, genetic, and immune-based
approaches) for the treatment and prevention of HIV.
There are three lines of evidence that suggest Sickle
Cell Hemoglobin (SCH) has a protective effect against HIV acquisition/
progression. First, anecdotal observations by investigators at Rutgers
NJMS noted the above, which suggested lower transmission rates of PHIV
among HIV exposed neonates with SCGA compared with those without [5]. Second, an epidemiological study by Nouraie et al. [8]
documented a two-fold lower risk of HIV infection and comorbidity in
patients with SCD among African-American adult patient hospital
discharges. This effect was observed over a 13-year period in over
400,000 patient records. Further, the authors of this article offered
several possible mechanisms for the apparent impact of SCGA on the
course of HIV disease including up-regulation of heme oxygenase-1,
hypoxia related to the anemia, higher expression of inflammatory
cytokines, inhibition of HIV transcription in the presence of reduced
iron, as well as use of hydroxyurea in SCD patients. Third, SCGA is
already documented to have protective effects against other infectious
diseases, such as Malaria. Although this conferred protection occurs by
conformational changes in the hemoglobin molecule by the SCGA inhibiting
the penetration and proliferation of the Malaria Parasite in RBCs,
there may be other characteristics of this gene allele product,
expressed on lymphocytes and other white blood cells that may also
confer protection against HIV attachment, penetration and/or
proliferation [9].
A possible mechanism of protection could exist within
the genome of patients with SCGA. Past literature has already
elucidated the mechanism by which certain Caucasians of Northern
European descent have conferred resistance to HIV [10].
These persons possess a 32-base-pair deletion on the CCR5 white blood
cell surface protein, one of two chemoreceptors utilized by HIV- 1 to
enter the leukocyte. The CCR5Δ32 mutation found in these individuals
results in a dysfunctional surface protein, preventing the virus from
entering the host [11].
In the case of individuals with SCGA, the authors further propose that
the TRlM5α protein could play a role in the protective effect. This
protein disassembles and degrades viral capsid binding, preventing the
pre-integration complex from arriving at the nucleus, thus interfering
with reverse transcription [12]. A study in 2004 conducted by Stremlau et al. [13]
identified TRlM5α as the blocking factor responsible for blocking HIV-1
strains from infecting Old World Monkeys13. Subsequent research has
shown that rhesus TRlM5α (TRlM5αrh) appears to be more stable, and thus more potent, than human TRlM5α (TRlM5αhu),
lending a reason as to why the majority of humans are immune to HIV-1
infection. Examination into the effect of various polymorphisms in
TRlM5αhu revealed differing frequencies of HIV infection
among African American cohorts. The TRlM5α 136Q polymorphism-indicating a
glutamate at residue 136 in the TRlM5α protein - was identified as
possessing a potential protective effect, as it was found at a greater
frequency in the High-Risk Exposed Uninfected African American
population [14].
Furthermore, the TRlM5α gene locus is found in close proximity to the
HBB gene locus on human chromosome 11, lending the possibility that
linkage disequilibrium interaction between the two could exist. Because a
large proportion of African American individuals possess the SCGA, the
authors further propose that a potential link between SCGA and TRlM5α
136Q may explain the lower-than-expected proportion of HlV-infected
individuals with the SCGA.
While a recent systematic review concluded that SCD
slows the progression of HlV into AlDS, and conversely HlV infection
complicates the course of patients with SCD [15],
no well controlled study of a relationship between the presence of SCGA
and the acquisition or progression of HIV infection has been conducted.
Such a study would identify the sickle cell status (SS vs. SC vs. SA
vs. AA) in HlV-infected cohorts and, conversely, the HlV status in
identified sickle cell cohorts. The population of HlV-infected patients
who carry the SCGA would be the target population of a prospective study
to determine the impact associated with the comorbidity of HlV
infection and SCGA status. The high prevalence of SCGA and HlV infection
in the Sub-Saharan African population and the preliminary observations
of a possible impact of SCGA status and HlV incidence as well as the
progression of HlV-1 disease, justifies further study of the possible
interaction between these two significant global health problems. The
results of such a study would have a major impact on the management and
follow-up of individuals followed in these countries as well as
encourage collaboration with and within these resource-constrained areas
of the world to address the global health challenges experienced by
much of the world's population.
Acknowledgement
Barry Dashefsky MD, Bart Holland PhD, MPH, François
Dabis MD, MPH, Nathalie De-Rekeneire MD and Said Aboud MD are thanked
for their contributions to this work.
Author Contributions
JO made original observation that SCD appeared in his
population of PHIV infants to be underrepresented. HS assisted in
recruiting international support for this hypothesis. JM consulted on
presence of PHIV in her large cohort of SCD patients. AS provided
technical support in data evaluation. KS, GG, JK, RM, PP, AD, OF, and FD
provided technical support in data analysis and manuscript preparation.
For more articles in Academic Journal of
Pediatrics & Neonatology please click on:
https://juniperpublishers.com/ajpn/index.php
https://juniperpublishers.com/ajpn/index.php
Comments
Post a Comment