Gullian – Barre Syndrome Variant with Unilateral Facial Weakness-Juniper Publishers
Juniper Publishers-Journal of Pediatrics
Abstract
It is a postinfectious polyneuropathy due to
alteration of protein component of myelin (p2 neurotogenic peptide)
leading to demyelination because of autoimmune mechanism. Neurological
manifestation begins after 2 to 4 weeks of viral or bacterial infection.
Clinical expression includes an acute onset symmetrical ascending
weakness (both proximal & distal) with unilateral facial weakness
and respiratory weakness and autonomic dysfunction. The diagnosis
depends on clinical picture, electrophysiological findings and CSF
examination. Immunotherapy is the main stay of treatment. IVIG &
Plasmaphrersis done within 2 to 4 weeks of symptoms onset is
recommended. Treatment is warranted in non ambulatory patient (Modified
Hughes GBS disability scale). The patient who hav not responded to
initial IVIG treatment may benefit from second course of IVIG. General
supportive care includes cardiorespiratory care, physiotherapy,
nutritional management, management of neuropathic pain, bladder–bowel
care and prevention of deep vein thrombosis.
Keywords: Acute
inflammatory demelinating polyradiculoneuropathy (AIDP); Acute motor
axonal neuropathy (AMAN); Acute flaccid paralysis; Clinical
neurophysiology; ImmunotherapyAbbreviations: AIDP: Acute Inflammatory Demelinating Polyradiculoneuropathy; AMAN: Acute Motor Axonal Neuropathy; NCS: Nerve Conduction Study; IVIg: Intravenous Immunoglobulin
Introduction
Gullian – Barre syndrome is also known as acute
inflammatory demyelating polyradiculoneuropathy (AIDP), acute motor
axonal neuropathy (AMAN). AIDP is the predominant subtype in North
America & Europe while AMAN is commonly reported subtype in Asia
including India & Central & South America. About 65% of children
report preceding upper respiratory tract & gastrointestinal tract
infection. Immunopathogenesis involve molecular mimicry & formation
of cross reacting antiganglioside antibodies. The GBS has several
variants depending upon distribution of motor, sensory, cranial,
autonomic or cebellar involvement variant, among them AIDP is the most
common [1]
Discussion
Clinical expression
- Muscle pain, difficulty while walking or refusals to walk are often the first presenting symptoms (50%)
- Distal limb weakness which ascending & symmetrical (20%) Areflexia.
- Respiratory muscle weakness & facial weakness (20%)
- Sensory symptoms including painful parathesia, backache & meningismus (50% -80% )
- Transient bladder involvement can occur in few children.
- Approximately 25% develop respiratory insufficiency requiring artificial ventilation & 75% have autonomic dysfunction. The course is monophasic in most of the children. 80 % reach maximum severity within 2 weeks & 97 % in 4 weeks.This phase is followed by a relatively static ‘plateau phase’ ranging from 2 days to 6 months before recovery begins [1].
Diagnosis
The diagnosis of GBS is clinical & is supported
by a few investigations. Characteristic CSF finding are of Albumino-
Cytological dissociation i.e. a combination of elevated CSF protein
& normal cell count. Nerve conduction studies help in diagnosis of
different sub types of GBS. In early phase of GBS motor & sesory
nerve conduction study (NCS) is normal. In such situation diagnosis
supported by prolonged F waves latencies. NCS abnormalities tend to peak
by 2 weeks of illness. Children with GBS should be
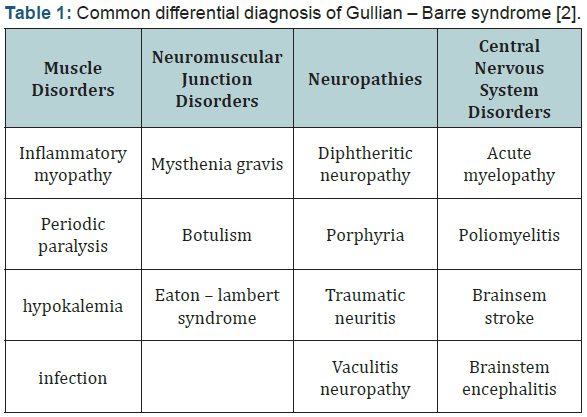
managed in PICU during initial phase (Table 1) [2,3].
Clinical neurophysiology
Earliest abnormalities is a drop in the amplitude of the evoked
muscle action potential & conduction blocks. Marked slowing of
nerve conduction can be recorded in about 50% patients. Reduced
compound motor unit potential amplitude is the most frequent
finding. Absence or prolongation of F wave is common. Proximal
blocks can be detected by measuring F wave. Conduction studies
improve slowly over a period of several months. Spontaneous
fibrillation may detected on electromyography during recovery
phase after 2 or 3 weeks [1].
Pathogenesis
AIDP is multifocal noninfective inflammatory process causing
demyelinaton or axonal degeneration of peripheral nerves. ADIP is
generally due to T cell mediated immune myelin damage. Increased
incidence of several axonal degeneration in GBS following C. jejuni
infection is known with more severe involvement. The mechanism
of axonal damage is different molecular mimicry due to shared
epitopes with gangliosides [1] (Figure 1).
Prognosis
Recovery is common & often complete in the majority.Mortality
is now ≤ 5%. Most of the death in childhood are due to preventable
respiratory complication. Acute axonal neuropathy, with good
pgognosis.Acute axonal and sensory axonal neuropathy generally causes poor prognosis. Miller Fisher variant in which cerebellar
signs, cranial nerves are involved also has a poor prognosis. The
disabilities included foot drop, pes cavus and postural tremor &
persisting weakness of the hands [1].
Treatment
Supportive symptomatic treatment is the mainstay of
therapy in the majority. The child should be closely observed in
hospital, objective assessment of respiratory function, regular
measurement of vital capacity is performed. Ventilatory support
should be considered if there is evidence of respiratory insuffiency
[3,4].
- Dysphagia if present necessitates nasogastric feeding.
- Chest and limb physiotherapy should be initiated early and carried out carefully. Bladder and bowel function should be attended to.
- Plasmapheresis has been shown to be effective in decreasing severity and shortening the non ambulatory phase. No significant complication ensue fron well conducted plasmapheresis. Several paediatric studies support the results.
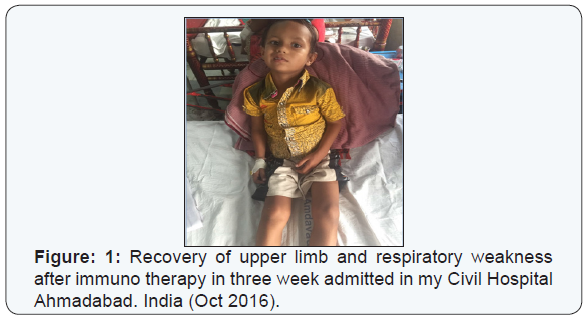
- Intravenous immunoglobulin (IVIg) is atleast as effective as Plasma exchange. IVIg is now become the preferred treatment due to ease of administration. Dose of IVIg 0.4 g/ kg body weight daily given on five successive days or two successive doses of 1 g/kg may be given active treatment of impending/ respiratory failure is imperative. Indication of immunotherapy includes a hughes GBS disabilities scale ≥3 or when patient is unable to walk unaided for 10 meters [5-7].
Conclusion
Acute flaccid paralysis in children is a medical emergency.
AFP is a clinical syndrome with array of differential diagnosis.The
common causes of AFP are Gullian – Barre syndrome, Anterior
horn cell myelitis and Acute transverse myelitis. Rapid evolution
of the weakness can lead to respiratory failure. Hence a child
with AEP Should be managed in PICU in the initial few days.
Immunotherapy is a main stay treatment.
For more articles in Academic Journal of
Pediatrics & Neonatology please click on:
https://juniperpublishers.com/ajpn/index.php
https://juniperpublishers.com/ajpn/index.php
Comments
Post a Comment