Evaluation of the Effects of Multiple Transfusions on Lipid Peroxidation in Preterm Infants-Juniper Publishers
Juniper Publishers-Journal of Pediatrics
Abstract
Aim: Preterm infants commonly
receive at least one packed red blood cell transfusion during their stay
in the neonatal intensive care unit and even though the complications
related to prematurity are multifactorial, especially bronchopulmonary
dysplasia, necrotizing enterocolitis and intraventricular hemorrhage
have been shown to be independently related with red blood cell
transfusions. This study aims to determine the extent of lipid
peroxidation in preterm babies after multiple transfusions, and to
figure out acut-off value of ferritin to start or withhold iron
prophylaxis in transfused babies.
Methods: Serum malondialdehyde and
ferritin levels of 23 preterm babies (born earlier than 35th gestational
week) were measured and the relation between total number of red blood
cell transfusions, malondialdehyde and ferritin levels were assessed.
Results: The relations between the
transfusion numbers and ferritin levels; and, ferritin and
malondialdehyde levels were statistically significant. Another
noteworthy finding was that the increase in serum malondialdehyde levels
was significantly higher in infants with serum ferritin levels >450
ng/mL.
Conclusion: It is important to
monitor serum iron status and lipid peroxidation in preterm babies who
were multiply-transfused. Ferritin and malondialdehyde levels can be
used for this monitorization.
Keywords: Red blood cell transfusion; Malondialdehyde; Ferritin; PretermIntroduction
Preterm infants, especially those smaller than 29
weeks of gestation and birth weight less than 1000 gr, often receive at
least one red blood cell (RBC) transfusion during their stay in the
neonatal intensive care unit (NICU) mostly for the treatment of anemia
of prematurity [1-3]. The anemia of prematurity is caused by multiple
factors; the most common being blood loss due to clinical testing and
diminished bone marrow production caused by decreased erythropoietin
levels and low iron stores [1,4]. Many studies show correlations between
transfusions and complications of prematurity (i.e. bronchopulmonary
dysplasia, necrotizing enterocolitis, retinopathy of prematurity, and
intraventricular hemorrhage) [5-7].Even though these complications are
multifactorial and the smaller, more unstable babies are more likely to
receive transfusions, especially bronchopulmonary dysplasia (BPD),
necrotizing enterocolitis (NEC) and intraventricular hemorrhage (IVH)
have been shown to be independently related with RBC transfusions
[8-11].
During preparation and storage of packed RBC, changes
occur in the cells called “storage lesion”. Adenosine triphosphate and
2,3-diphosphoglycerate decrease, potassium increases and oxidative
changes occur [11]. Considering that all the antioxidant molecules and
mechanisms are decreased in premature neonates, these babies are prone
to oxidative damage. Moreover, transfusion-mediated iron load and
especially non-transferrin bound iron may contribute to this oxidative
damage and prematurity complications [1,11,12].
Oxidative status can be evaluated in plasma by
measuring actors of oxidative stress (free radicals and their
metabolites), their products such as modified biomacromolecules,
products of lipid peroxidation such as malondialdehyde (MDA), and
changes in the concentrations of antioxidant enzymes and molecules [13].
The primary aim of this study was to determine the
relationship between blood transfusions, ferritin levels and lipid
peroxidation in preterm infants. Secondary aims were to monitor
iron overload, find the cut-off level after which oxidative damage
becomes significant and also to determine a cut-off value to start
or withhold iron prophylaxis in transfused babies.
Materials and Methods
The study was designed as a cross-sectional pilot study.
Twenty-three preterm infants whose gestational ages were
smaller than 35 weeks were enrolled. Their demographic data and
transfusion numbers are summarized on tables 1 and 2. Restricted
transfusion guidelines were used to decide the necessity of
transfusions [14]. Venous blood samples were randomly taken
when they were at least 20 days of age in a period free of infection
according to clinical signs and laboratory test results (negative
C-reactive protein and procalcitonin levels and sterile blood
cultures). Serum MDA levels were measured by high performance
liquid chromatography (HPLC) (Ultimate 3000, ThermoDionex,
USA) with a flourescence detector. Within-run precision values
were 1.8-5.5% and between-run precision values were 6.5-9%
for 0.40-1.55 μmol/L MDA, according to manufacturer’s claim.
The lower detection limit was 0.02μmol/L. The method was
adapted from the one applied by Hageman, et al. [15]. Serum
iron and iron binding capacity were measured colorimetrically
(Cobas 8000 Modular Analytics, Roche Diagnostics, Germany).
Ferritin levels were measured with an immunometric test with electrochemiluminescence detection (Modular Analytics E170,
Roche Diagnostics, Germany).
SPSS version 16.0 was used to analyze the results. Pearson
correlation test was used for normally distributed data and a
Spearman’s rho for nonparametric data. The level of significance
was accepted as p0.05. To find a cut-off level for ferritin
concentration which lead to a significant MDA increase, the ferritin
limit to withhold iron prophylaxis defined by World Review of
Nutrition and Dietetics (300μg/L) [16] was taken as a starting
point and ferritin levels were compared by grouping the values
with 50μg/L increments. Mann-Whitney U test was used to find
the level of significance among MDA levels.
This study has been approved by the ethics committee of our
institution.
Result
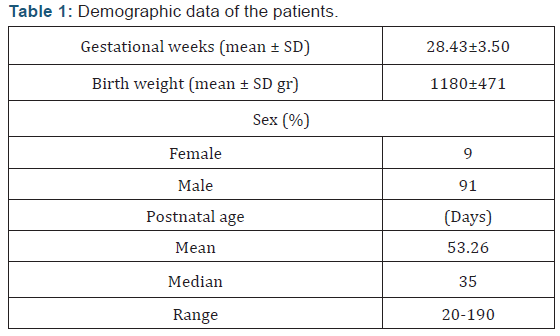
Twenty-three preterm infants with a mean gestational age
(±SD) 28.43(±3.50) weeks; birth weight 1180(±471) g were
included in the study. Two of the patients (%9) were female and
21 of them were male (%91) (Table 1). Five of the babies (group 0
- 21.7%) were never transfused, while 10 cases (group 1 - 43.5%)
were transfused less than 5 times, 2 cases (group 2- 8.7%) 6-10
times, and 6 cases (group 3 - 26.1%) were transfused more than
10 times. The median transfusion number was 16 (range: 1-33),
the patient who was transfused for 33 times was a preterm with
IVH, BPD and NEC needing multiple operations and prolonged
periods of high frequency oscillatory ventilation.
There was a significant difference in serum ferritin levels
between transfused (median: 457 ng/mL, range: 108-2717) and
non-transfused (median: 203ng/mL, range: 102-268) infants
(p=0.017). There was a statistically significant correlation
between serum ferritin and MDA levels (p0.001; r=0.693). Also,
the correlation between the number of transfusions and serum
ferritin levels was statistically significant (p=0.016; r=0.558).
Serum MDA levels were significantly higher in infants with serum
ferritin levels >450 ng/mL (p0.001), which coincides with the 97th
centile of normal ferritin levels designated by Obladen, et al [17].
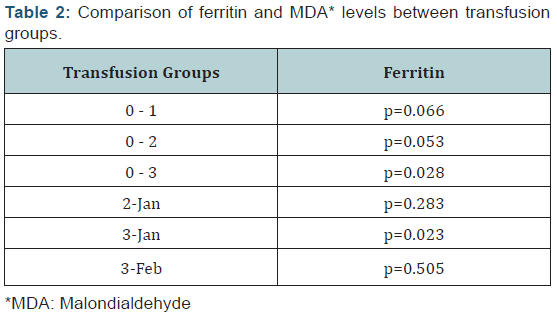
When transfusion groups were compared, there was a statistically
significant difference between ferritin levels of groups 0 and 3 –
which was to be expected since group 0 was never transfused – and
between ferritin and MDA levels of groups 1 and 3 (Table 2). When
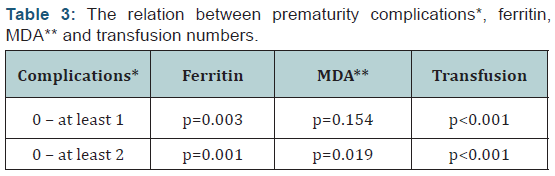
the infants were grouped according to the number of prematurity
related complications (those who had no complications, who had
one complication and those who had two or more complications);
transfusion numbers, serum ferritin, and MDA levels of those
with two or more complications were significantly higher when
compared to cases without complications (p0.001, p=0.001, and
p=0.019, respectively) (Table 3).
Discussion
Almost all of extremely low birth weight infants receive RBC
transfusion during their hospitalization period. However, after
multiple transfusions serum iron and ferritin concentrations
increase [4]. After transfusion, non-transferrin bound (NTB)
iron and MDA increase in the plasma of preterm babies [18,19].
Ferritin contributes to the damage by releasing NTB iron during
oxidative stress [4]. Therefore, it is necessary to monitor iron
and oxidative status of these babies. In our study, we have shown
that there is a statistically significant correlation between ferritin
and MDA levels. Significant correlation was also present between
ferritin levels of the patients and the times they were transfused.
The number of transfusions has been associated with
prematurity-related complications [1-3]. In our study, the babies
who suffered from at least two complications had significantly
higher ferritin and MDA levels and their transfusion numbers
were significantly higher. Monitoring iron status is also important
for determining the time of iron prophylaxis. Elemental iron
should be given to non-transfused babies to prevent anemia [4].
Even though there are many guidelines for supplemental iron, it is
still not clear when to start this prophylaxis in transfused babies
with high ferritin levels [16-20]. It is advised by World Review of
Nutrition and Dietetics [16] that iron supplementation should be
withheld until ferritin level falls below 300μg/L, which coincides
with the 90th centile of normal levels of ferritin designated by
Obladen, et al. [17]. In our study, we have shown that MDA levels
raise significantly in preterm babies whose ferritin levels are
above 450μg/L, which coincides with the 97th centile of normal
ferritin levels.
The sophisticated assays and complicated methods to detect
the free iron responsible of oxidative damage bear need to find easy
and reliable tests to evaluate oxidative stress – though indirectly
– in babies with multiple transfusions. Taking its limitations as
an acute phase reactant into consideration, in patients without
acute inflammation ferritin levels can reflect lipid peroxidation
as we have shown its correlation with MDA, the levels of which
were higher in infants with two or more prematurity-related
complications.
Conclusion
In conclusion, iron status of very low birth weight infants has
to be monitored to detect iron deficiency and also transfusionrelated
iron overload. It is important to use restrictive transfusion
guidelines in order to protect preterm from iron overload and
oxidative stress [14]. Ferritin can be used to assess the iron status
of preterm, until better parameters and easier assays are found
to indicate oxidative stress. As this was a pilot study consisting
of 23 preterm neonates - the biggest limitation of our study –
further research is necessary to determine a cut-off level for
ferritin to decide when to start iron prophylaxis, especially in
cases with high ferritin levels. If further studies confirm these
results, it would be relevant to move the ferritin cut-off limit for iron replacement initiation from 300μg/L to 450μg/L – where
oxidative stress effects are more prominent. Instead of withholding
iron prophylaxis from those who have ferritin levels >300μg/L,
more babies could receive iron therapy and those who would have
otherwise been transfused at least one more time, might have a
chance to rise their hemoglobin levels, saving themselves from a
few more possible transfusion-associated side effects.
For more articles in Academic Journal of
Pediatrics & Neonatology please click on:
https://juniperpublishers.com/ajpn/index.php
https://juniperpublishers.com/ajpn/index.php
Comments
Post a Comment